Blood biomarkers for lung cancer have raised great expectations in their clinical applications for early diagnosis, prognosis, and therapeutic responses. However, low abundance and poor specificity of these conventional serum markers, such as carcinoembryonic antigen (CEA) and squamous cell carcinoma antigen (SCC), have hampered their implementation. Various tumor types, including lung, release exosomes into the blood that are enriched with DNA, reflecting the mutational status of the originating cancer cell, mRNA, miRNA and proteins, with important roles in disease onset, progression and metastasis. Thus, exosome derived biomarkers provide a promising avenue for early lung cancer diagnoses and improved prognosis. However, existing exosome purification technologies (ultracentrifugation, multi-step filtration, antibody conjugated magnetic beads or polyethylene glycol based precipitation) present great challenges for clinical implementation. They are expensive, time-consuming, require large sample volumes and frequent manual handling, and result in poor yields and unpredictable purity of exosome preparations, especially when derived from clinical plasma samples. To overcome these critical hurdles, we propose to develop a cost-effective, innovative Exosome Total Isolation Chip (ExoTIC) to isolate large amounts of pure exosomal preparations from a wide range of clinical bio-fluids, which can be easily implemented in the clinic. The ExoTIC device provides a promising route to discover exosome-based novel biomarkers for the early diagnosis and monitoring of disease progression in lung cancer.
Researchers at Stanford have developed an inexpensive, rapid and efficient method to isolate a high yield of pure exosomes from a wide range of clinical biofluids. Exosomes are small (30-180nm) cell-derived vesicles that are shed into bodily fluids such as blood, urine and saliva. Those that are derived from tumor cells contain biomarkers such as tumor specific proteins and nucleic acids that are indicative of cancer stage and progression. Thus circulating, tumor-derived exosomes have great promise as biomarkers for noninvasive cancer detection. However, current exosome isolation methods are expensive, tedious, non-standardized and often result in poor yields and impure preparations. To overcome these limitations the inventors have developed this device, the exosome-total-isolation-chip (ExoTIC), to isolate tumor specific exosomes. This simple, easy-to-use, size-based filtration device will allow rapid, high yield and high purity isolation of exosomes from a wide range of bodily fluids. These exosomes can then be used to identify and map nucleic acid alterations and mutations, and associated protein molecules. This technology provides a promising tool for the development of exosome-based biomarkers for early cancer detection, prognostication and treatment monitoring.
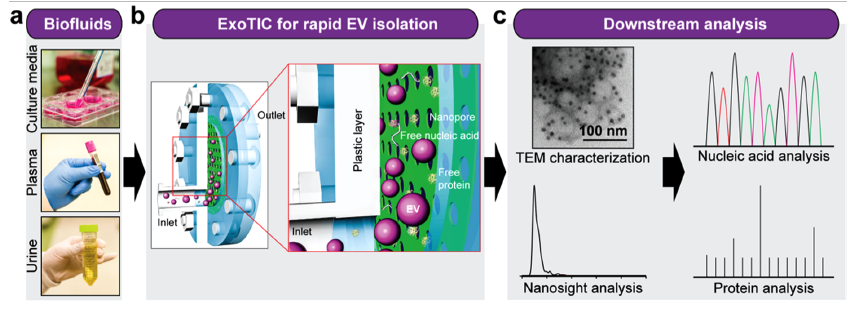
a. Various biofluids (culture media, plasma, urine, etc.) can be processed for exosome isolation. b. Schematic of size-based isolation using the ExoTIC device. Intact exosomes are enriched and purified at the filter. c. Different downstream analysis that can be performed on the isolated exosomes.
